Covid-19 Vaccine Information
NOTE: As of May 15th, 2021, the information in this section will no longer be updated. We invite you to visit the CDC’s website to find the most up-to-date information related to the COVID-19 vaccines.
Do you have any questions about the COVID-19 vaccines? We’ve assembled answers to many frequently asked questions below.
Clinical trials on the effectiveness of vaccines on children are ongoing, we will add the information as soon as it becomes available to the public.
Esta información también esta disponible en español, aqui.
The vaccines
What COVID vaccines are available in the US?

Three vaccines for COVID are currently available, Pfizer, Moderna, and Johnson & Johnson vaccines. Because the vaccines were safe and effective, the FDA gave these vaccines emergency use authorization.
- Read the most updated Moderna COVID Vaccine Fact Sheet (from FDA) – see “Download EUA Fact Sheet for Recipients & Caregivers”
- Read the most updated Pfizer COVID Vaccine Fact Sheet (from FDA) – see “Fact Sheet for Recipients & Caregivers”
- Read the most updated Johnson and Johnson (also called Janssen) COVID Vaccine Fact Sheet (from FDA) – see “EUA Fact Sheet for Recipients and Caregivers”
What does the vaccine cost to me?
The vaccine and injection are free of charge to you. The vaccine is paid for by the US government for everyone. For people that are insured, the cost of the injection is fully covered by public or private insurance companies. For people that are uninsured, the injection is covered by the Health Resources and Services Administrations Provider Relief Fund.
What should you know about the Johnson & Johnson/Jassen vaccine?
This section will be updated as more information becomes available to the public. Last updated on 4/15/21.
What is the lastest update on the Johnson & Johnson (J&J) vaccine?
- On April 13th, 2021, the CDC and FDA made an announcement to pause the use of the Johnson & Johnson COVID-19 vaccine. This is a reasonable measured response and indicates that the government’s health surveillance system is working. Although the pause may come as a surprise, it is a natural part of the scientific and public health processes.
Should I be worried about this update?
If you have gotten a J&J shot already, there is absolutely no reason to panic, especially if it’s been more than 3 weeks since your dose.
The recommendation to pause J&J vaccine distribution comes as six women ages 18-48 reported experiencing a blood clot within two weeks of getting the shot. It is still unknown whether the blood clots and the vaccine are related. This pause is in place to provide time for further investigation and tells us that the government is vetting these events closely, as they should. It also alerts doctors to watch for clots and informs on management standards.
6.5 million Americans have received the J&J vaccine so far, making these six cases extremely rare. Rare complications are possible with many drugs and vaccines. The CDC and FDA are taking the appropriate steps to review the facts to provide the safest recommendations as we move forward.
What should I do if I received the J&J vaccine?
If you received the J&J vaccine and have experienced symptoms including severe headaches, abdominal pain, leg pain, or shortness of breath within three weeks of your injection, please contact your healthcare team.
What happens next?
While this pause is a bump in the road as the authorities work to get vaccines out to as many people as possible quickly, it is not a reason to panic. It will allow the authorities to learn new information so we are able to best advise and treat patients who MAY develop these very rare clots.
In the meantime, it is critical we continue to follow all risk mitigation protocols (wearing masks and social distancing) and not lose sight of the huge benefits of the vaccines to helping us end this pandemic.
Who should get the vaccine?
I’ve heard that younger people might not get very sick from COVID-19, so why is it important that I still get the vaccine?
- While it is true that older people have a higher risk of dying from a COVID infection, young people can get very sick or even die when infected with COVID-19, whether or not they have underlying risk factors. Also, young people can get long lasting effects (“long haul COVID”) from COVID even if the initial infection is not severe. Additionally, even if you are lucky enough to not getany symptoms from COVID, you can still infect others who are vulnerable to getting very sick.

If I already was infected with COVID virus, should I still get the vaccine?
- Yes! Even if you were infected with COVID previously, current recommendations are to get vaccinated. We do not know how long the immunity lasts from a COVID viral infection.
Who can get the vaccine?
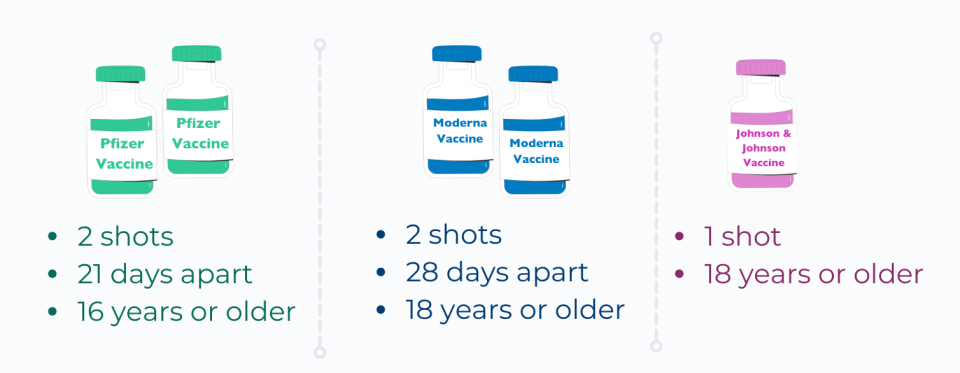
- The Pfizer vaccine has been approved for people 16 and older
- The Moderna vaccine has been approved for people 18 and older
- The Johnson and Johnson vaccine has been approved for people 18 and older
There are currently no vaccines allowed for use in teens and children under 16 years old. Clinical trials for children as young as 12 are ongoing, and trials for children younger than 12 are in development.
Are there any medical conditions that make it not ok to get a COVID vaccine?
If you had a bad reaction to the first dose of the vaccine (like difficulty breathing or another severe allergic response) you shouldn’t get another dose. (Moderna info, Pfizer info, Johnson and Johnson info)
- Read the most updated Moderna COVID Vaccine Fact Sheet (from FDA) – see “Download EUA Fact Sheet for Recipients & Caregivers”
- Read the most updated Pfizer COVID Vaccine Fact Sheet (from FDA) – see “Fact Sheet for Recipients & Caregivers”
- Read the most updated Johnson and Johnson (also called Janssen) COVID Vaccine Fact Sheet (from FDA) – see “EUA Fact Sheet for Recipients and Caregivers”
Is the COVID vaccine safe for people who are pregnant or planning to become pregnant?
Yes, both the Pfizer and Moderna vaccines are recommended by the American College of Obstetrics and Gynecology and CDC for people who are pregnant or planning to become pregnant and are eligible for vaccination. This recommendation is based on animal safety studies, as well as monitoring of people who became pregnant during the clinical trial. The safety of the vaccine has not been studied specifically in pregnant or breastfeeding people, but these trials are planned and underway. Also, people who are pregnant and get the vaccine can choose to register for the completely voluntary v-safe registry, the goal of which is to get information about the health of people who are pregnant and get the COVID vaccine.
There was a false rumor that the COVID spike protein that these vaccines target was similar to a protein involved in placental growth and attachment called syncitin-1. The false rumor said that because of this similarity the vaccine posed a risk to pregnancy. This rumor is not true, the spike protein is different than the syncitin-1 placental protein, and is not believed to negatively impact pregnancy.
Why should I get the COVID vaccine?
The vaccine protects you from getting very sick from COVID-19. People who become infected with COVID-19 can get very sick or even die. In fact, over 500,000 people have died in the US alone from COVID this past year.
We want to protect teachers and students to create a safe learning environment! This involves:
- Following local and federal recommendations for safe learning practices, remote or in-person.
- Working with departments of health, teachers unions, and school administrators.
- Taking standard COVID precautions (mask-wearing, 6’ distancing, washing hands) and offering vaccines to teachers.
Scientists don’t know if the COVID vaccine reduces the risk of infecting other people with COVID virus in addition to protecting you. This is why it is very important to keep following standard COVID precautions (face mask, 6’ distancing, hand washing, avoiding crowds).
If I am more suspectable to getting COVID-19 due to comorbidities, should I still get the vaccine?
Yes, for most people who have underlying health issues, getting the vaccine is not only safe but extra important since you are at higher risk of becoming severely ill if you get infected with COVID. If you have a weakened immune system, an autoimmune condition, have had a bad allergic reaction in the past, or have concerns about how another health condition that you are concerned about definitely reach out to a health care provider to get answers to your questions.
How does the COVID vaccine works?
Is the COVID vaccine safe?
- Yes, the COVID vaccine is safe and effective. Based on clinical trials of tens of thousands of volunteers, both vaccines were determined to be safe for most people. Also, between December 2020 to March 2021, over 92 million COVID vaccine doses were safely given to the general public.
- The people in the clinical trials and the general public who have gotten the vaccine continue to be monitored for side effects. Learn more about the vaccine development, testing, and approval process here.
- The vaccines met strict safety rules and have been shown to be safe and work in adults of all ages, races, and sexes (Pfizer, Moderna, Johnson & Johnson).
- A very rare side effect of the vaccine is a severe allergic reaction that can make it difficult to breathe. Clinicians can treat this with a medicine called epinephrine. This allergic reaction happens right after getting the vaccine, which is why you will be observed for 15 minutes after getting vaccinated.
- People can report vaccine side effects through the FDA and CDC Vaccine Adverse Event Reporting System (VAERS) at 1-800-822-7967 or at https://vaers.hhs.gov/reportevent.html. Regulators are monitoring these systems to pick up on any patterns of worrisome side effects or new side effects.
- The bottom line is, based on what we know, the benefits of getting the vaccine outweigh the risks for most people.
How effective are the vaccines?
- All of the vaccines protect against symptomatic and severe cases of COVID (such as being hospitalized) if you were to get infected.
Does the vaccine give you COVID-19?
- The Pfizer, Moderna, and Johnson & Johnson vaccines cannot give you COVID because they do not contain the COVID virus. The vaccines work by telling your body to make a small piece of the surface of the COVID virus so that your body’s immune system can learn to identify it and stop it.
How is the vaccine given?
- Pfizer – 2 shots given in the arm, 21 days apart
- Moderna – 2 shots given in the arm, 28 days apart
- Johnson and Johnson – 1 shot given in the arm

Why do I need to get 2 doses for the Pfizer and Moderna vaccines?
What are the side effects of the vaccine?
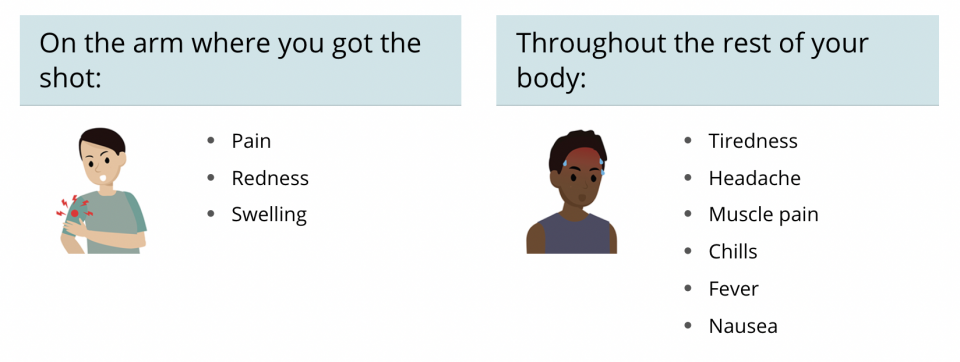
- Common side effects are: injection site soreness, fatigue, headache, muscle and join pain, fever. You can treat these symptoms by drinking fluids, getting plenty of rest, and taking ibuprofen (such as Advil or Motrin) or Acetaminophen (such as Tylenol). These side effects will get better within about 2 days. These side effects are more likely after the second dose.
- Rare side effect: severe allergic reaction within 1 hour of getting the injection. You will be monitored for 15 minutes after getting the shot for this reaction, which can be reversed with medication.
What about the long term side effects of the vaccine?
- There are not any currently known long term side effects of the Pfizer, Moderna, or Johnson and Johnson vaccines. The FDA will continue to monitor bad side effects.
How much immunity do I have after each dose of the vaccine and when do I have the maximum protection?
- The Pfizer vaccine gives about 46% protection against getting COVID 2 weeks after the first dose, and about 92-95% protection against COVID 2 weeks after the 2nd dose. The Moderna vaccine gives about 95% protection 2 weeks after the 2nd dose (studies haven’t looked at protection after only 1 dose only, however, we would expect it to be similar to the Pfizer vaccine since they both use mRNA). You are considered fully vaccinated 2 weeks after you get the 2nd of two doses for both Pfizer and Moderna, and 2 weeks after you get the one Johnson & Johnson vaccine dose.
- The Johnson and Johnson vaccine gives about 66% protection against moderate to severe COVID 28 days after getting the injection. You are considered fully vaccinated 28 days after you get the first (and only) dose.
How long does the COVID vaccine protect against the disease?
- We do not know how long the vaccine will protect you yet. This research is ongoing. We do know that it will protect you more than not getting the vaccine though.
How was it made?
Was the vaccine tried in a racially and ethnically inclusive group of people?
- The Pfizer, Moderna, and Johnson & Johnson clinical trials included people who identified as Black (9.8% in Pfizer, 9.7% in Moderna, and 17.2% in Johnson & Johnson), Asian (4.4%, 4.7%, 3.5%), and American Indian/Alaska Native (0.6%, 0.8%, 8.3%), Native Hawaiian or Other Pacific Islander (0.2%, 0.2%, 0.3%), and White (81.9%, 79.4%, 62.1%). Further, people identifying as Hispanic made up 26.2% of the Pfizer trial group and 20.0% of the Moderna trial group, and 45.1% of the Johnson & Johnson trial group.The vaccines met strict safety rules and have been shown to be safe and work in adults of all ages, races, and sexes (Pfizer and Moderna).
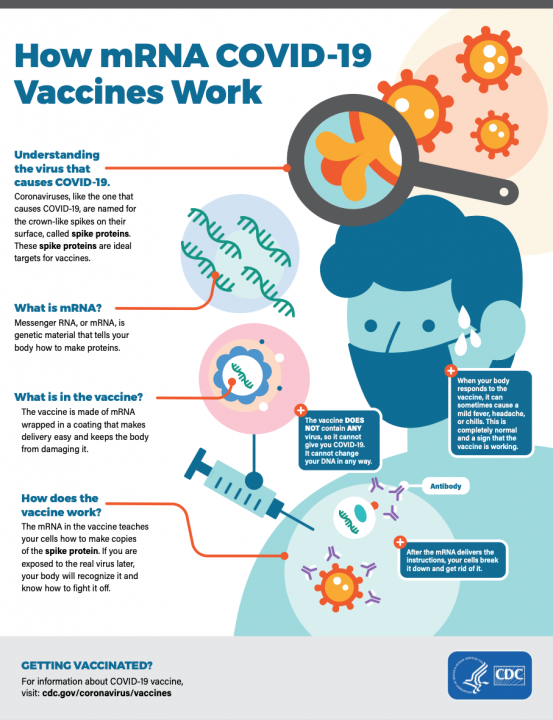
How does the vaccine work?
- Both the Pfizer and Moderna vaccines use messenger RNA (also called mRNA), to make a mug shot of one of the proteins on the COVID virus to teach your immune system make to recognize and attack the COVID virus if you were ever to be infected with it, something that is naturally made by your body.
- The Johnson & Johnson vaccine uses a harmless non-COVID virus to make a mug shot of the COVID virus in your cells so that your body learns how to get ready to fight against COVID.
Can the mRNA vaccines affect my DNA?
- No mRNA vaccines do not affect your DNA, in fact, mRNAs are so weak and unstable at room/body temperature they have to be stored at really cold temperatures. Once the mRNA has been injected into your body they only last a short time to help your immune system out, before your body breaks them down.
If the vaccine approval process was so short, was this just rushed through?
- While the vaccine approval process was very efficient, the FDA and scientists that developed the vaccine were very careful to follow safety checks and protocols. There are a few reasons why the vaccine process was about to move so quickly:
- Scientists had already studied similar coronaviruses before COVID, so they already knew a good amount about the virus
- Adenovirus based (Johnson and Johnson) and mRNA based (Pfizer and Moderna) vaccine technologies had already been used before COVID
- Because of the urgency of needing a vaccine, scientists put a lot more time and energy into the vaccine development process, and countries like the US poured a lot of money into the process to make sure that the development could be done well and efficiently.
- The FDA used an Emergency Use Authorization, which still requires robust safety data but allows for drugs to move through the regulatory process more efficiently than usual
What is the difference between a vaccine and a treatment?
- Vaccines prevent disease, so you need to get the vaccine before getting sick in order for it to be effective. Treatments on the other hand are used once someone has gotten sick and do not prevent illness.
What to expect after vaccination?
Will life be back to normal after getting vaccinated?
Even after being vaccinated, you need to make sure to follow all the standard COVID guidelines – wearing a mask, avoiding large groups, keeping 6’ distance, washing hands. However, you will know that you and our community is one step closer to a COVID-safer world! One day, once enough people have gotten vaccinated and the levels of the virus are much lower, we can return to a safer and more normal existence.
The Pfizer and Moderna clinical trials included people who identified as Black (9.8% in Pfizer and 9.7% in Moderna), Asian (4.4%, 4.7%), and American Indian/Alaska Native (0.6%, 0.8%), Native Hawaiian or Other Pacific Islander (0.2%, 0.2%), and White (81.9%, 79.4%). Further, people identifying as Hispanic made up 26.2% of the Pfizer trial group and 20.0% of the Moderna trial group. After you are fully vaccinated (2 weeks after your second dose or a 2-dose vaccine or 2 weeks after your 1-dose vaccine, you can start to do some things that aren’t recommended for unvaccinated people. For example, you can gather indoors with small groups of other fully-vaccinated people without a mask. The CDC is learning more about what’s safe while vaccination levels are still rising. You can read their guidance about what you can do after vaccination here.
What are booster shots, do I need them?
- The need for booster shots is not currently established. The idea behind booster shots is that they could help improve the immune response for people already vaccinated against virus variants and/or any decline in immune response over time.
Additional information
Where can I find reliable information on COVID and COVID Vaccines?
Be a savvy reader! Here are some other resources to help guide your research and questions as you learn more to make this important decision.
- English CDC COVID Resources
- English WHO COVID Resources
- English Maryland COVID LINK
- English FDA COVID 19 Information
- Multilingual COVID-19 Resources (from WHO)
- Multilingual COVID-19 Resources (from FDA)